VSEPR Answer Key
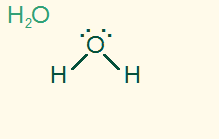
tetrahedral
bent 109.5°
AB2E2, sp3
2σ 2 LP, polar
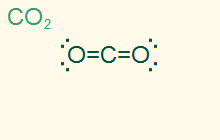
linear
linear
AB2, sp
2σ 2 π, nonpolar
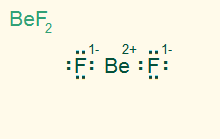
linear
linear
AB2, sp
2σ, ionic
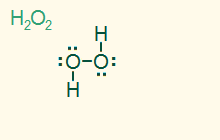
tetrahedral
bent 109.5°
AB2E2, sp3
2σ 2 LP, polar
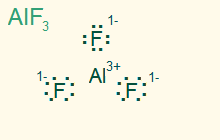
trigonal planar
trigonal planar
AB3, sp2
3σ, ionic
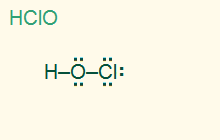
tetrahedral
Cl – O is linear
AB1E3, sp3
1σ 3 LP, polar
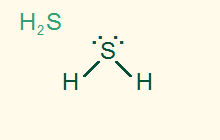
tetrahedral
bent 109.5°
AB2E2, sp3
2σ 2 LP, polar
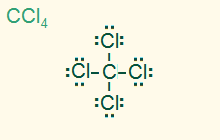
tetrahedral
tetrahedral
AB4, sp3
4σ, nonpolar
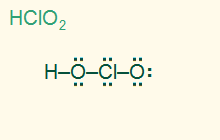
tetrahedral
bent 109.5°
AB2E2, sp3
2σ 2 LP , polar
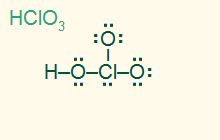
tetrahedral
trigonal pyramid
AB3E, sp3
3σ 1 LP, polar
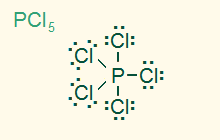
trigonal bipyramid
trigonal bipyramid
AB5, sp3d
5σ, nonpolar
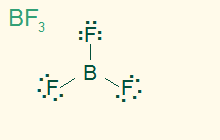
trigonal planar
trigonal planar
AB3, sp2
3σ, nonpolar
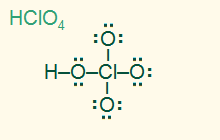
tetrahedral
tetrahedral
AB4, sp3
4σ, polar
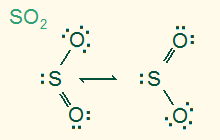
trigonal planar
bent 120°
AB2E, sp2
2σ 1π 1 LP, polar
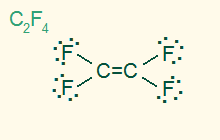
trigonal planar
trigonal planar
AB3, sp2
3σ 1π, nonpolar
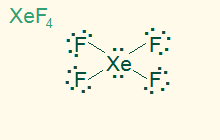
octahedral
square planar
AB4E2, sp3d2
4σ 2 LP, nonpolar
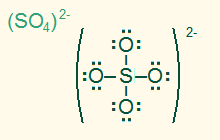
tetrahedral
tetrahedral
AB4, sp3
4σ, ionic
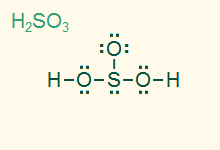
tetrahedral
trigonal pyramid
AB3E, sp3
3σ 1 LP, polar
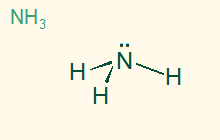
tetrahedral
trigonal pyramid
AB3E, sp3
3σ 1 LP, polar
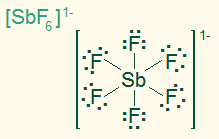
octahedral
octahedral
AB6, sp3d2
6σ, ionic